Scaling from within-host infection dynamics to between-host transmission
In this paper, Handel and Rohani discuss quantitative links that need to be made to bridge scales of within-host dynamics of immune response and pathogen load, to pathogen transmission dynamics at the population level. For example, pathogens and immune response interact to influence pathogen load and host symptoms. These latter factors can influence host infectiousness, and in combination with host behavior, these factors then affect pathogen spread from infected to susceptible individuals.
More specifically, pathogen load (measured either instantaneously or as an integrated value) can positively affect transmission. However, the time course of an infection (which can refer to its duration, as well as the trajectory of the pathogen load over time) is important for determining the transmission potential of a pathogen. This is particularly true in acute diseases. These diseases tend to have a shorter duration because their pathogen load rapidly progresses to high levels that lead to either rapid host death or to a strong immune response that clears the infection more quickly than in less acute diseases. A shorter infection duration means that an infected host has a shorter time frame in which it can successfully pass the infection on to susceptible hosts. Thus, pathogen load over time and infection duration can interact to influence pathogen transmission.
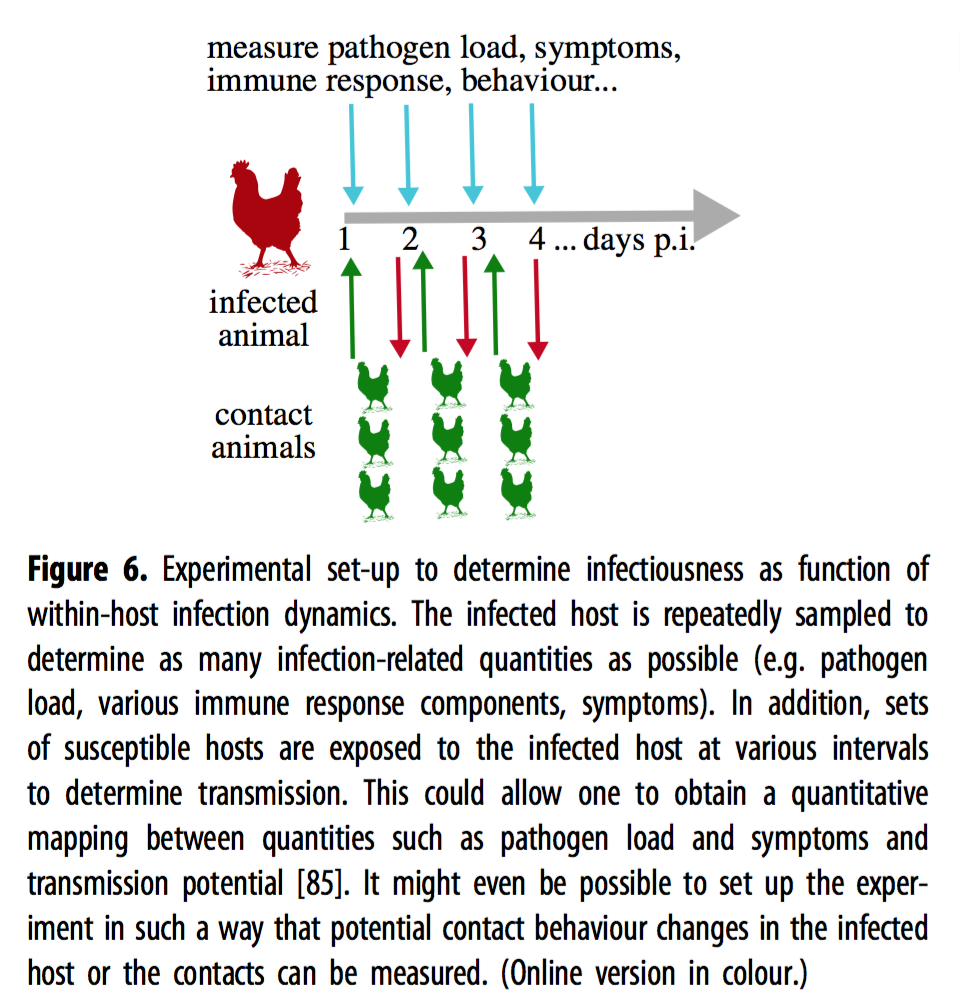
Host symptoms can further modify transmission. For example, increased nasal discharge in conjunction with a high pathogen load might increase the transmissibility of a respiratory pathogen. To account for the effects of symptoms on transmissibility, the authors suggest expressing symptoms as a function of pathogen load, and then using this approximation of infectiousness to model transmission. However, this approach may only be feasible in limited cases, as immune response and other factors may modulate the relationship between symptoms and pathogen load. For most pathogens, acquiring more data to characterize these relationships will be necessary for research to be able to use this approach. The authors illustrate an experimental design that would allow researchers to quantitatively describe within-host infection dynamics that influence transmission in Fig. 6.
Host behavior can further modify transmission, either enhancing or reducing it, depending on how the infection influences the host’s likelihood of contact with susceptible individuals, as well as the timing of these contacts in conjunction with pathogen load and symptom expression. Understanding how these latter factors feed back to influence host behavior may help link within-host processes to between-host transmission. This knowledge will improve our ability to model transmission dynamics for specific pathogens and their effects at the larger population scale, which is important for the development of more effective strategies for reducing disease spread.
Handel, A. & Rohani, P. 2015. Crossing the scale from within-host infection dynamics to between-host transmission fitness: a discussion of current assumptions and knowledge. Philosophical Transactions of the Royal Society B: Biological Sciences 370, 20140302.